Difference between revisions of "Single Cell Scheduler"
Jump to navigation
Jump to search
(→Important Information) |
(→Important Information) |
||
| Line 3: | Line 3: | ||
==== Important Information ==== | ==== Important Information ==== | ||
| − | # This form ( | + | # This form ([https://help.igb.illinois.edu/images/1/12/10x_Single_Cell_Form.docx | 10x Single Cell Form.docx]), filled out completely. Email form to the lab ([mailto:ngsequencing@illinois.edu ngsequencing@illinois.edu]) prior to setting up your 10x experiment date. |
# Starting concentration should be at least 4,000 cells/ul (4M cells/ml) or more. We request 1M cells total, transported to us in a 1.5ml centrifuge tube. There are modified protocols for limited cells, please ask if this is your case. | # Starting concentration should be at least 4,000 cells/ul (4M cells/ml) or more. We request 1M cells total, transported to us in a 1.5ml centrifuge tube. There are modified protocols for limited cells, please ask if this is your case. | ||
# Elimination of dead cells is critical, viability should be >70%. Your starting cell suspension will be counted upon arrival with live/dead measure included. If viability is below 70%, you may wish to repeat cell collection as background RNA levels will be high. If we are asked to continue, cells with >70% viability will be processed through the Miltenyi Biotec Dead Cell removal and counted again, which could significantly reduce total cells depending upon dead fraction. | # Elimination of dead cells is critical, viability should be >70%. Your starting cell suspension will be counted upon arrival with live/dead measure included. If viability is below 70%, you may wish to repeat cell collection as background RNA levels will be high. If we are asked to continue, cells with >70% viability will be processed through the Miltenyi Biotec Dead Cell removal and counted again, which could significantly reduce total cells depending upon dead fraction. | ||
Revision as of 11:23, 6 March 2019
Contents
Contact Information[edit]
- All inquiries about 10x Single Cell should be directed to ngsequencing@illinois.edu.
Important Information[edit]
- This form (| 10x Single Cell Form.docx), filled out completely. Email form to the lab (ngsequencing@illinois.edu) prior to setting up your 10x experiment date.
- Starting concentration should be at least 4,000 cells/ul (4M cells/ml) or more. We request 1M cells total, transported to us in a 1.5ml centrifuge tube. There are modified protocols for limited cells, please ask if this is your case.
- Elimination of dead cells is critical, viability should be >70%. Your starting cell suspension will be counted upon arrival with live/dead measure included. If viability is below 70%, you may wish to repeat cell collection as background RNA levels will be high. If we are asked to continue, cells with >70% viability will be processed through the Miltenyi Biotec Dead Cell removal and counted again, which could significantly reduce total cells depending upon dead fraction.
- Once the process above is complete, final cell suspension should have at least 20ul total volume with a concentration between 700-1,200 cells/u* (700k -1.2M/ml).
- Each library will be made from 500-10,000 cells, depending upon your project needs. Doublets increase linearly with total cells, from ~0.6% at 1000 cells, to ~6% at 10,000 cells.
- Recommended target per sample is 3,000 cells or more. While libraries can be made to target 500-10,000 cells each, libraries capturing < 3,000 cells have much greater variability in actual cells captured (+/- 50% or more) and lower reproducibility. Variability of actual capture at or above 3,000 cells should be ~30% or less (ie: 2,700-3,300 for a 3,000-cell target). While all protocols for library preparation will be followed, due to differences in sample types submitted for 10x single cell, no guarantee is given that your cell type will behave according to typical performance.
- Prior to library setup, you will need to tell us below how many cells should be targeted for each library, and how many different samples you have.
Using Online Scheduler to Schedule Meetings, Test Counts, and Experiments[edit]
10x Single Cell Scheduler - https://www-app.igb.illinois.edu/singlecell/
Gaining Access to Online Scheduler[edit]
Email the completed form listed above (File:10x Single Cell Form.docx) to ngsequencing@illinois.edu. You will receive an email when you have access to login to the scheduler.
Scheduling an Initial Meeting[edit]
- An initial meeting is required for new researchers or if it is for a new cell type.
- Meeting to discuss introduction to 10x and project set-up.
- Initial meetings will take place in ERML 334.
- Use these directions to schedule a meeting:
- Login to the scheduler using your netID and AD password.
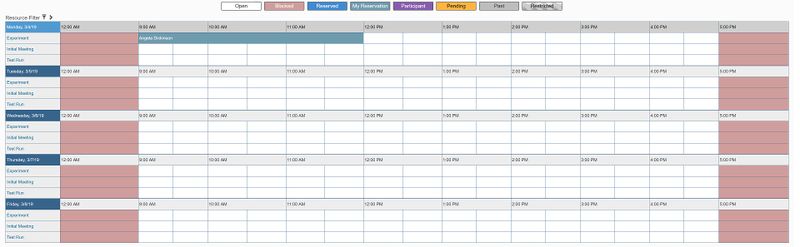
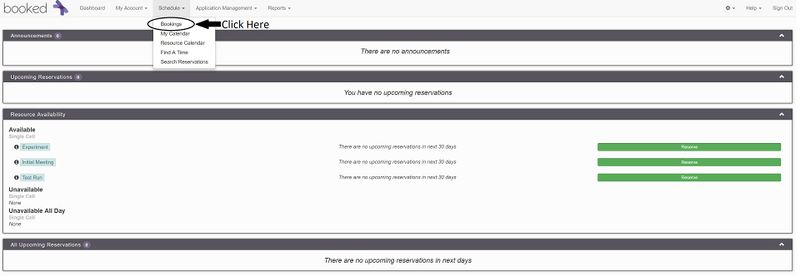
- Under "Schedule", click "Bookings"
- Next to "Initial Meeting", click on the time you would like to schedule a meeting.
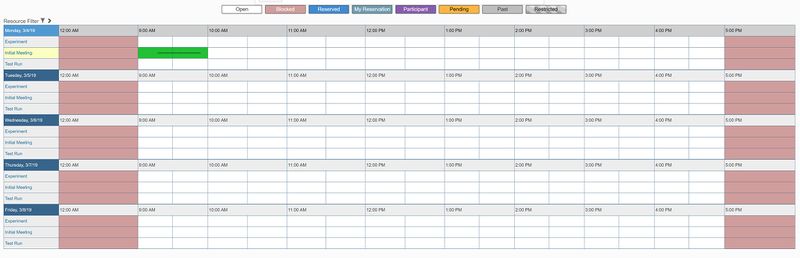
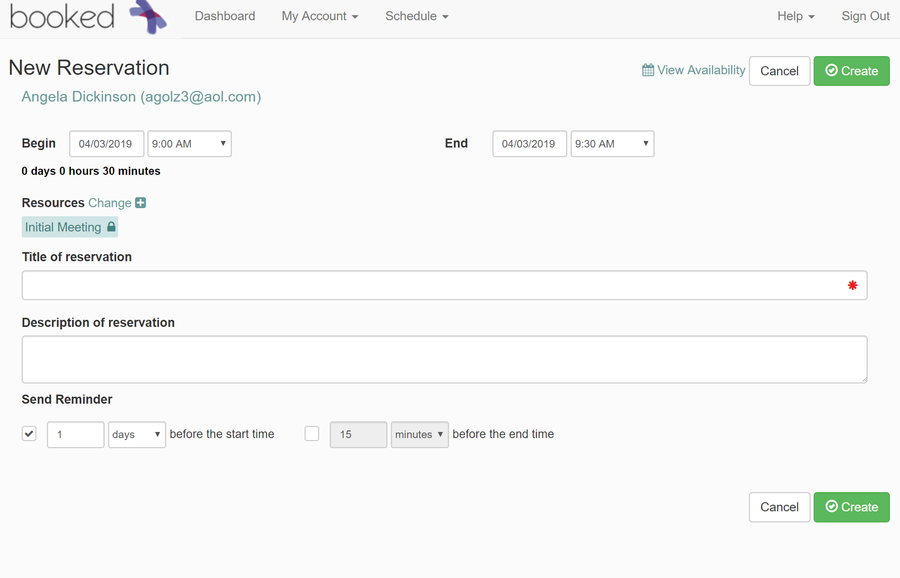
- A box will pop up to make your reservation. Enter the following information:
- Verify the date and time you would like your meeting to take place at.
- Under "Title of reservation", enter your first and last name.
- Under "Description of reservation", enter any additional comments you believe are needed.
- Click "Create"
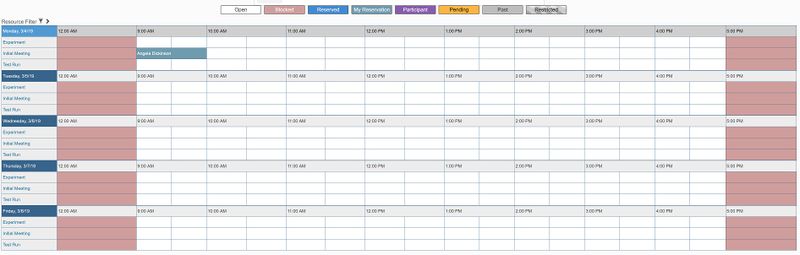
- You will receive confirmation of your reservation and should be able to see it on the calendar now.
Scheduling Test Counts[edit]
- There is no charge to run test counts.
- Any new cell type should have a test prior to the actual experiment.
- You must make your meeting at least 1 week in ahead of time.
- Make sure you schedule the appropriate amount of time for the number of cell types you have.
- 1-2 samples - 1 hour
- 3-4 samples - 1 hour and 30 minutes
- 4+ samples - 2 hours
- Test counts are performed in ERML 221.
- Login to the scheduler using your netID and AD password.

- Under "Schedule", click "Bookings"

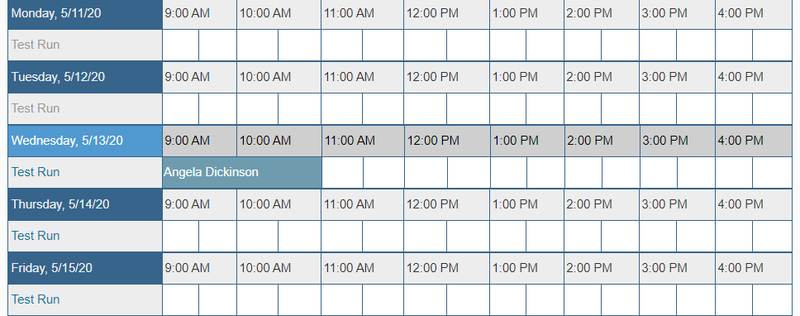
- Next to "Test Run", click on the time you would like to schedule a meeting.
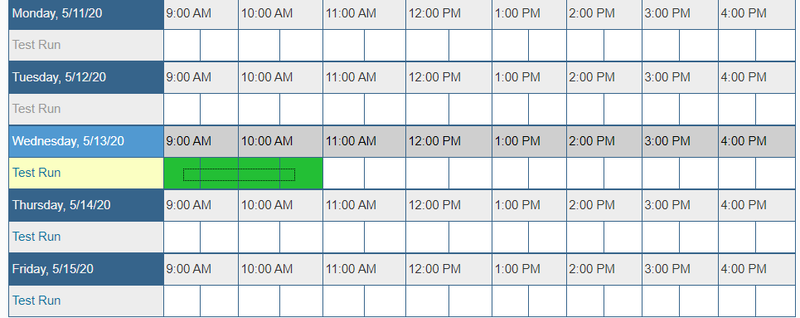
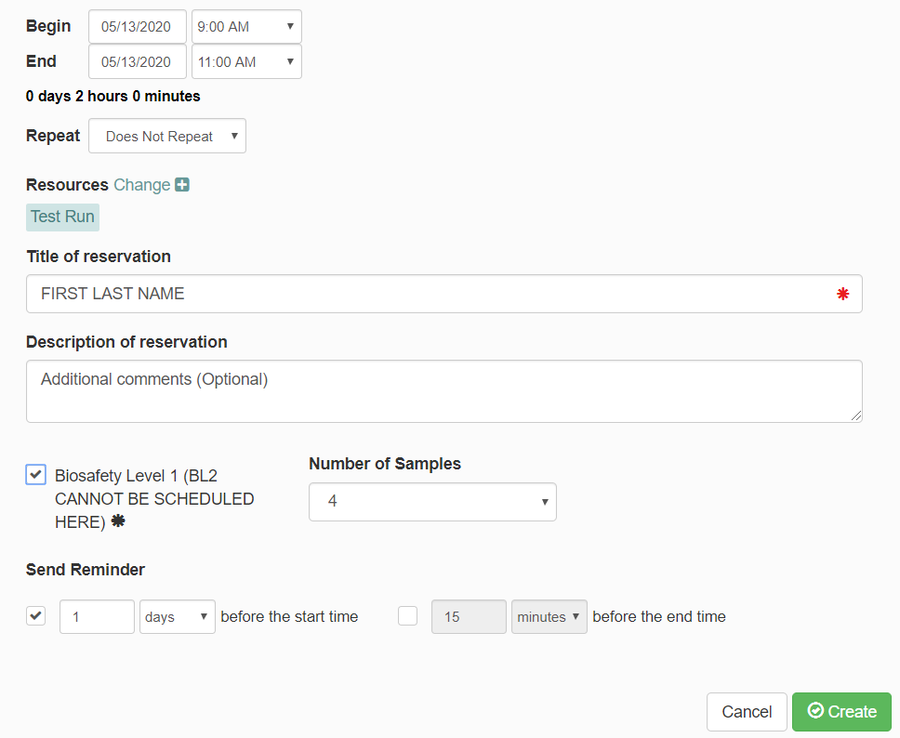
- A box will pop up to make your reservation. Enter the following information:
- Verify the date and time you would like your meeting to take place at.
- Under "Title of reservation", enter your first and last name.
- Under "Description of reservation", enter any additional comments you believe are needed.
- Under "Number of Samples", enter the number of samples you will be using (you can have a maximum of 16 for one experiment).
- Click "Create"
- You will receive confirmation of your reservation and should be able to see it on the calendar now.
Scheduling the Experiment[edit]
- You must schedule your experiment at least 1 week ahead of time.
- Make sure you schedule the appropriate amount of time for the number of cell types you have.
- 1-4 samples - 2 hours and 30 minutes
- 4+ samples - 3 hours
- Test counts are performed in ERML 221.
- Login to the scheduler using your netID and AD password.

- Under "Schedule", click "Bookings"

- Next to "Expiriment", click on the time you would like to schedule a meeting.
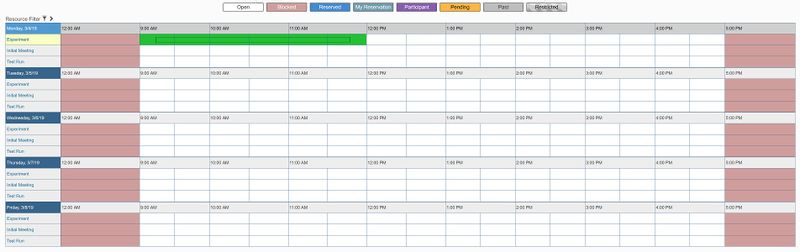
- A box will pop up to make your reservation. Enter the following information:
- Verify the date and time you would like your meeting to take place at.
- Under "Title of reservation", enter your first and last name.
- Under "Description of reservation", enter any additional comments you believe are needed.
- Under "Number of Samples", enter the number of samples you will be using (you can have a maximum of 16 for one experiment).
- Click "Create"
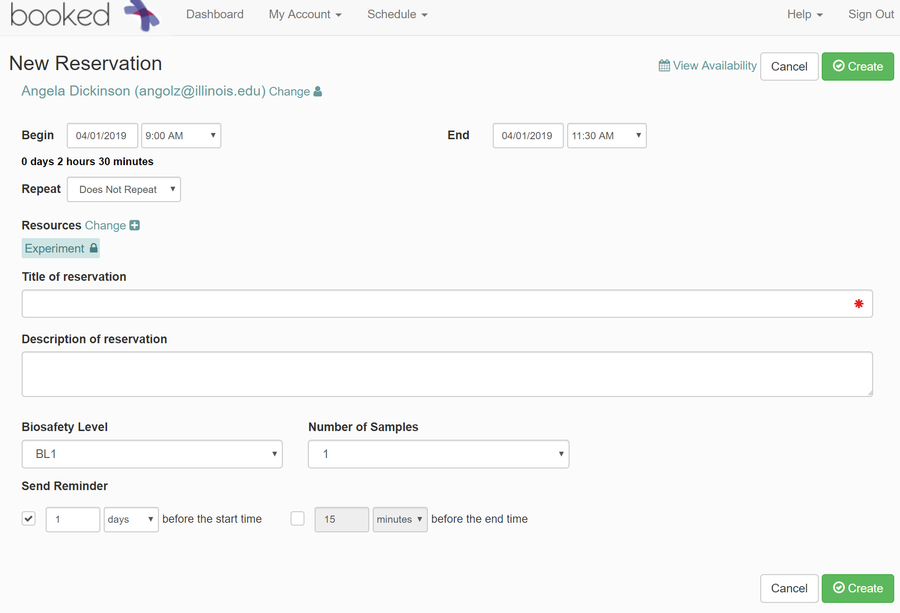
- You will receive confirmation of your reservation and should be able to see it on the calendar now.