Difference between revisions of "Single Cell Scheduler"
Jump to navigation
Jump to search
| (19 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | === | + | === DNA Services 10x Website === |
| − | * | + | *For more information, please visit the 10x website at this link: https://biotech.illinois.edu/htdna/applications/10x-submission |
| − | ==== | + | ===Instructions for Scheduling Test Counts=== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | *You can access the scheduler at this link: https://www-app.igb.illinois.edu/singlecell/ | |
| − | |||
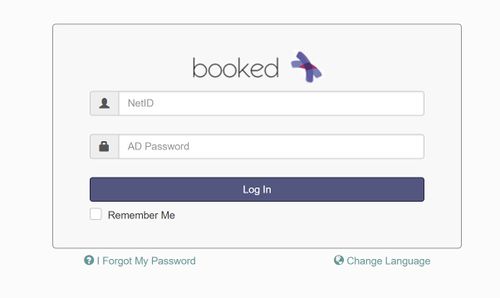
| − | + | * Login to the scheduler using your netID and AD password. | |
| − | + | [[File:Singlecell1.jpg|500px]] | |
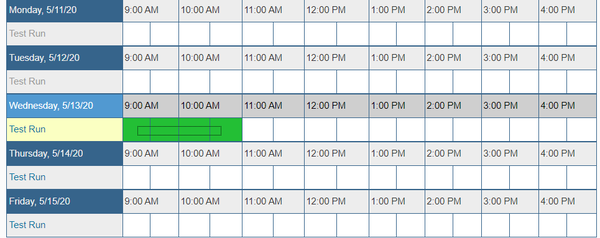
| − | + | * Next to "Test Run", click on the time you would like to schedule a test count. Note that if you have not been trained on using the counter, you need to contact the DNA Services facility to schedule an assisted test count. Please email them at [mailto:ngsequencing@illinois.edu ngsequencing@illinois.edu]. | |
| − | + | [[file:singlecell6.jpg|600px]] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
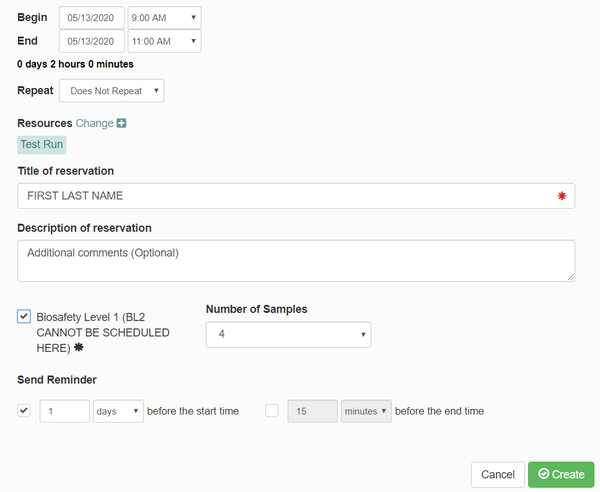
| − | + | * A box will pop up to make your reservation. Enter the following information: | |
| − | + | *** Verify the date and time of your meeting | |
| − | + | *** Under "Title of reservation", enter your first and last name. | |
| − | + | *** Under "Description of reservation", enter any additional comments you believe are needed. | |
| − | + | ***Check the box for BL1 samples. '''BL2 samples cannot be scheduled here. Contact [mailto:ngsequencing@illinois.edu ngsequencing@illinois.edu] to schedule BL2''' | |
| − | + | *** Under "Number of Samples", enter the number of samples you will be using. | |
| − | + | *** Click "Create" | |
| − | + | [[File:Testrun2.png|600px]] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | ***Verify the date and time of your meeting | ||
| − | ***Under "Title of reservation", enter your first and last name. | ||
| − | ***Under "Description of reservation", enter any additional comments you believe are needed. | ||
| − | ***Under "Number of Samples", enter the number of samples you will be using | ||
| − | ***Click "Create" | ||
| − | |||
| − | |||
| − | |||
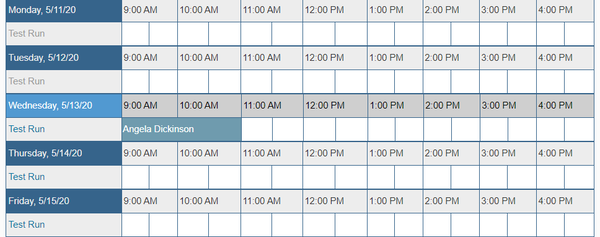
| − | + | * You will receive confirmation of your reservation and should be able to see it on the calendar now. | |
| − | + | [[File:singlecell8.jpg|600px]] | |
| − | + | ||
| − | + | *Please note, your reservation is for the Cell Counter in '''334 ERML''' | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Latest revision as of 12:19, 11 January 2021
DNA Services 10x Website[edit]
- For more information, please visit the 10x website at this link: https://biotech.illinois.edu/htdna/applications/10x-submission
Instructions for Scheduling Test Counts[edit]
- You can access the scheduler at this link: https://www-app.igb.illinois.edu/singlecell/
- Login to the scheduler using your netID and AD password.
- Next to "Test Run", click on the time you would like to schedule a test count. Note that if you have not been trained on using the counter, you need to contact the DNA Services facility to schedule an assisted test count. Please email them at ngsequencing@illinois.edu.
- A box will pop up to make your reservation. Enter the following information:
- Verify the date and time of your meeting
- Under "Title of reservation", enter your first and last name.
- Under "Description of reservation", enter any additional comments you believe are needed.
- Check the box for BL1 samples. BL2 samples cannot be scheduled here. Contact ngsequencing@illinois.edu to schedule BL2
- Under "Number of Samples", enter the number of samples you will be using.
- Click "Create"
- You will receive confirmation of your reservation and should be able to see it on the calendar now.
- Please note, your reservation is for the Cell Counter in 334 ERML